The Change In Montgomery-Asberg Depression Rating Scale (MADRS)



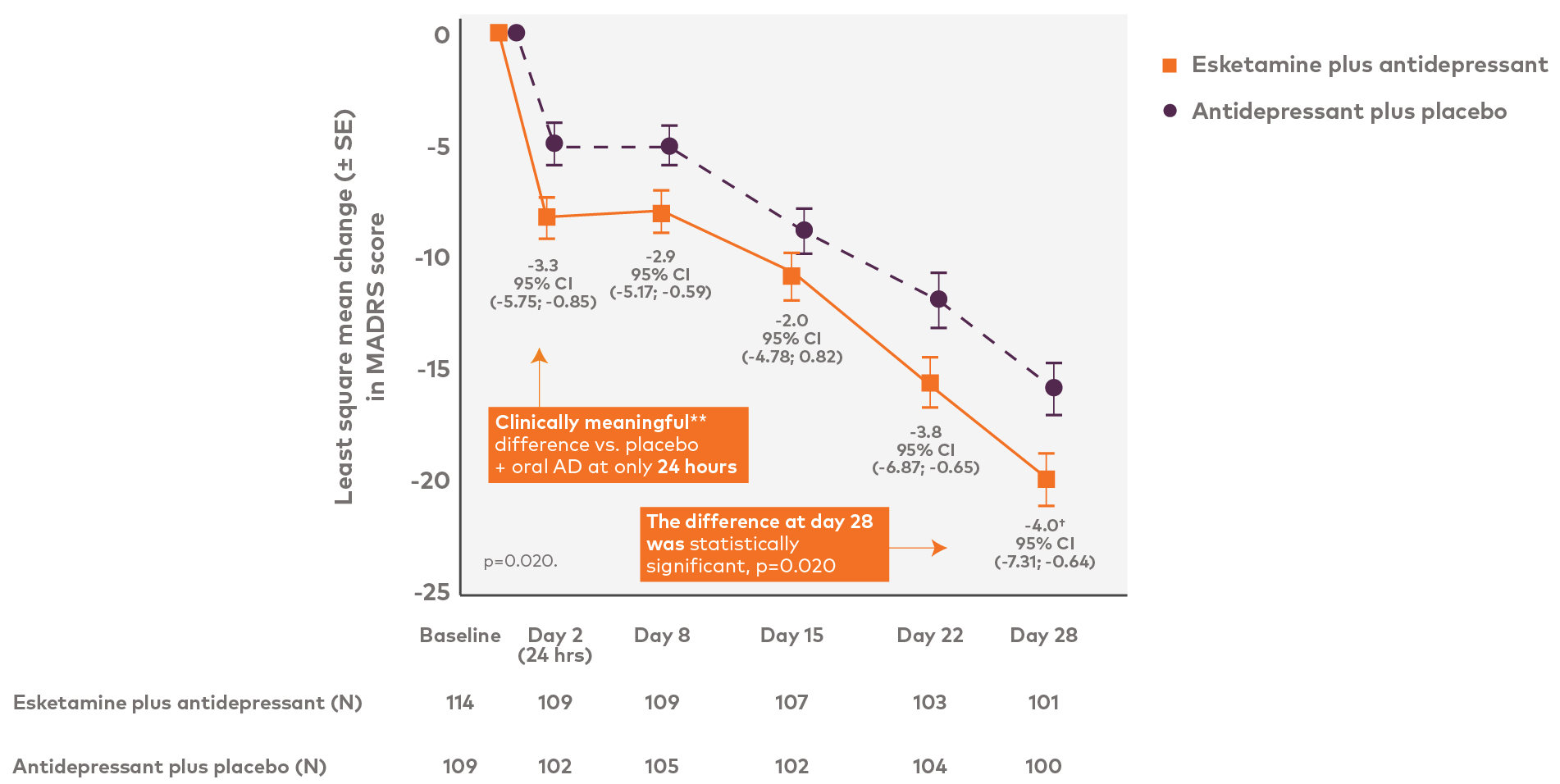
The primary efficacy endpoint, change in MADRS score from baseline (day 1) to endpoint (day 28) 1
* In adult patients (aged 18 to 64)1, † Response was rapid in onset and increased over time during repeated dosing with least square mean between-group differences, favoring Spravato,(-4.0, p=0.020 ) 1 TRD, Treatment Resistant Depression

Objective: About one-third of patients with depression fail to achieve remission despite treatment with multiple antidepressants. This study compared the efficacy and safety of switching patients with treatment-resistant depression from an ineffective antidepressant to flexibly dosed esketamine nasal spray plus a newly initiated antidepressant or to a newly initiated antidepressant (active comparator) plus placebo nasal spray.
Study Design: This was a phase 3 randomized, double-blind, active controlled, multicenter study conducted between August 2015 and November 2017. 223 patients (114 in the esketamine plus antidepressant group and 109 the antidepressant plus placebo group) randomly assign eligible patients in a 1:1 ratio to receive double-blind treatment with either esketamine (56 mg or 84 mg) nasal spray (hereafter referred to as esketamine) or placebo nasal spray (hereafter referred to as placebo), administered twice weekly, each combined with a newly initiated open-label oral antidepressant administered daily.
The Primary endpoint was change in MADRS score From baseline (day1) till Endpoint (day 28).
The mean MADRS score decreased from baseline to day 28, with greater improvement observed among those in the esketamine plus antidepressant arm as compared with the antidepressant plus placebo arm (difference of least square means=-4.0, SE=1.69, 95% CI=-7.31, -0.64; p=0.020)
Response was rapid in onset and increased over time during repeated dosing, with least square mean between-group differences, favoring esketamine, of -3.3 (95% CI=-5.75,-0.85) 24 hours after dosing.
MADRS,Montgomery–Åsberg, Depression Rating Scale
Popova V, Daly EJ, Trivedi M, et al. Efficacy and Safety of Flexibly Dosed
Esketamine Nasal Spray Combined With a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study. Am J Psychiatry 2019; 176 : 428-438.
Reference:
1- Popova V, Daly EJ, Trivedi M, et al. Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined With a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study TRANSFORM-2. Am J Psychiatry 2019; 176 : 428-438.
CP-225223