how to Administer Spravato
how to Administer Spravato


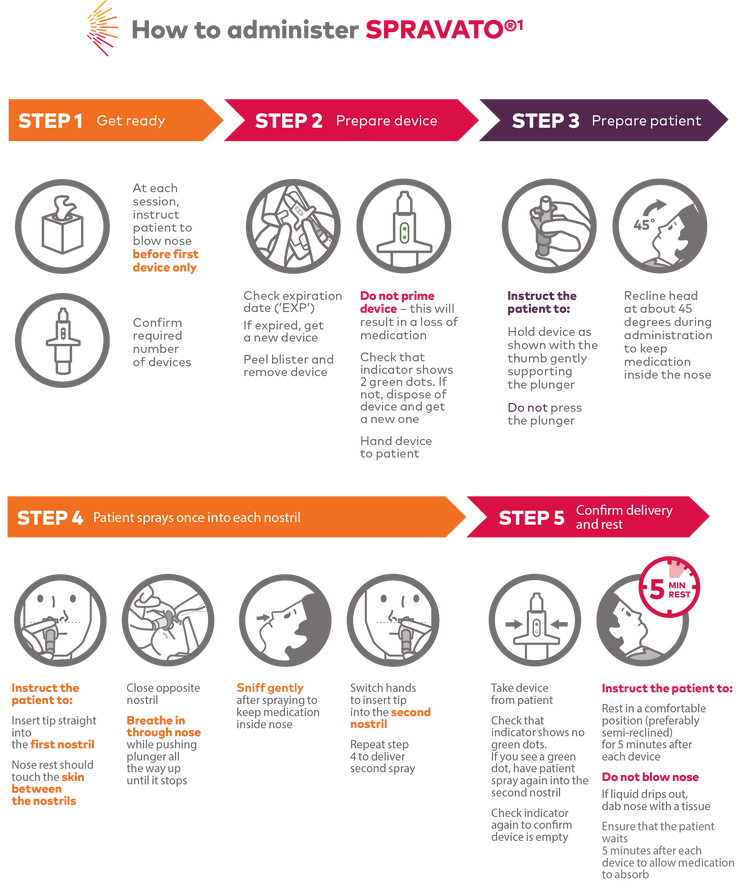
If sneezing occurs immediately after administration, or administration in the same nostril occurs, a replacement device should not be used.2
Reference:
1- Ministry of Health Spravato approved leaflet 13.7.2020.
2- Spravato Summary of Product Characteristics,https://www.ema.europa.eu/en
/documents/product-information/spravato-epar-product-information_en.pdf. Last Accessed 03.01.2021
CP-233067