
How can Simponi® help patients like W.E?

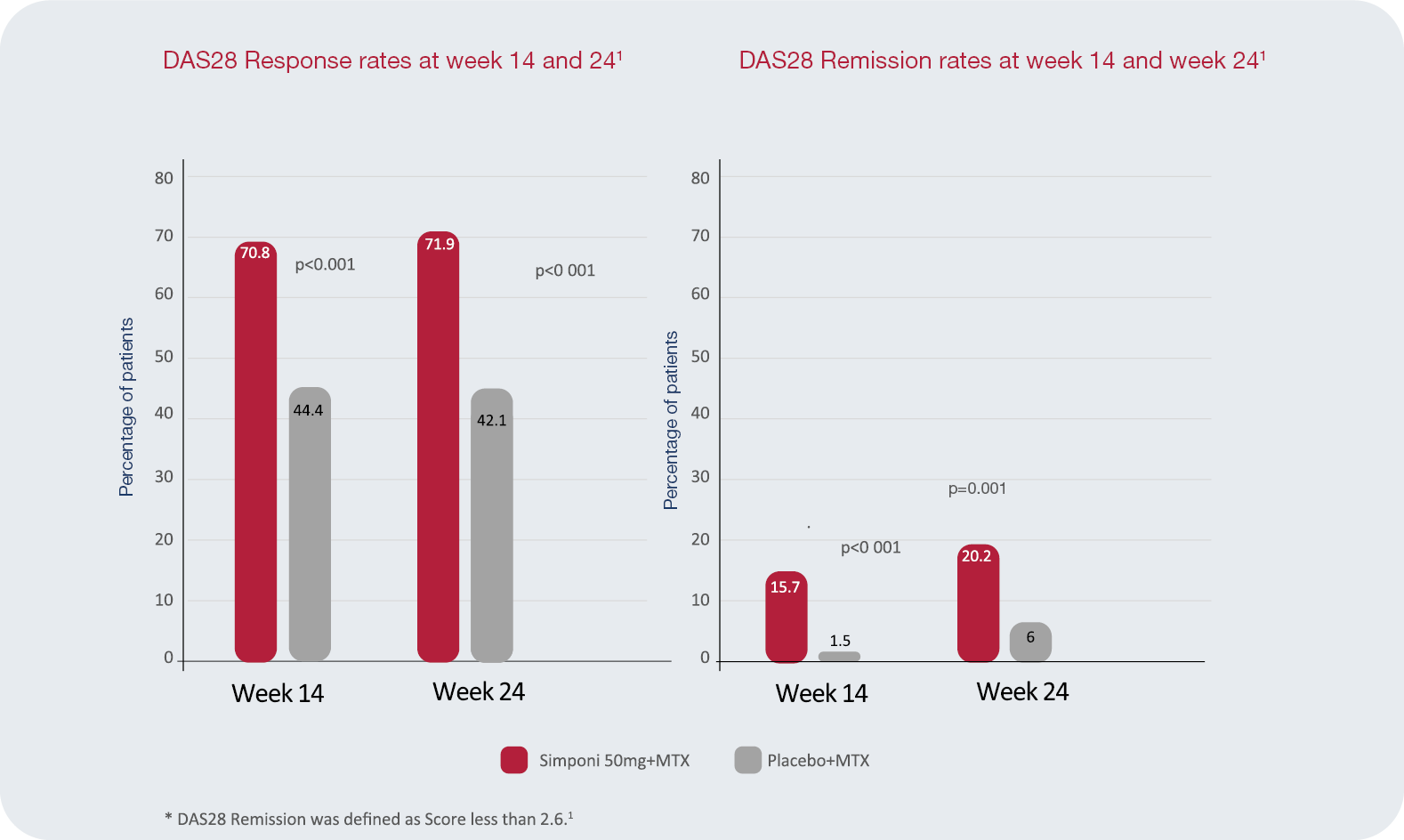
GO-Forward Study Details1
Objective: The phase III GO-FORWARD study examined the efficacyand safety of golimumab in patients with active rheumatoid arthritis (RA) despite methotrexate therapy.1
Study Design:This was a phase III, multicentre, randomised, double-blind, placebo controlled trial.The study included a double-blind controlled phase to week 52 and an open-label extension up to 5 years.1
Patients who still met the study criteria (n=444) were randomly assigned in a 3 : 3 : 2 : 2 ratio to receive: placebo injections plus methotrexate (group 1), golimumab 100 mg injections plus placebo capsules (group 2), golimumab 50 mg injections plus methotrexate (group 3), or golimumab 100 mg injections plus methotrexate (group 4).1
There were two co-primary endpoints: the proportion of patients achieving an ACR20 response at week 14 (p=0.001) and the improvement from baseline in HAQ-DI score at week 24 (p<0.001).1
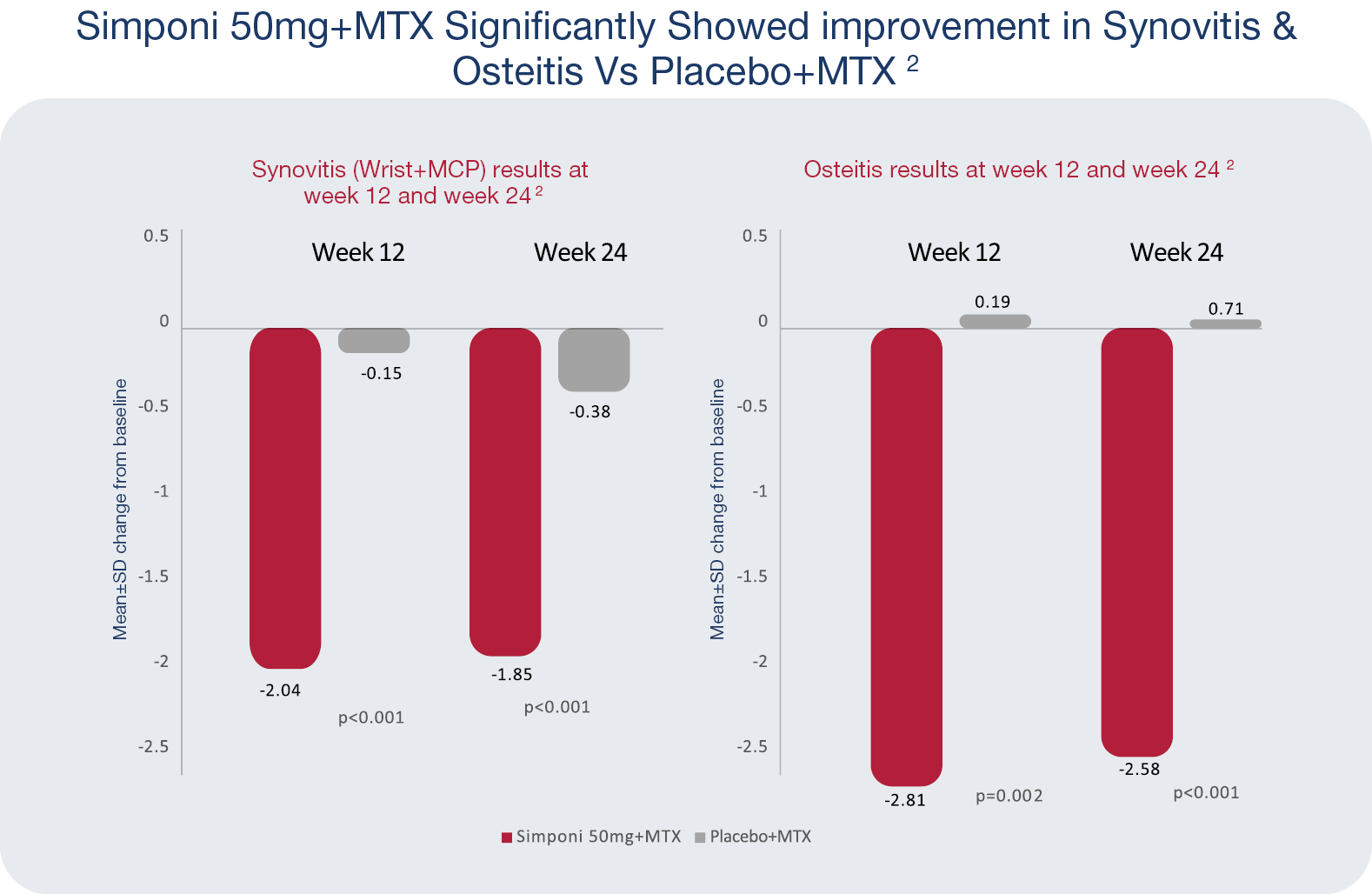
GO-Forward MRI Study Details2
Objective: To evaluate golimumab’s effect on MRI detected inflammation and structural damage in patients with active rheumatoid arthritis (RA) despite methotrexate (MTX).2
Study Design: A subset of the GO-FORWARD patients from eligible and willing sites participated in an MRI substudy (n=240). Patients were randomly assigned to receive placebo injections plus MTX capsules (group 1), golimumab 100 mg injections plus placebo capsules (group 2), golimumab 50 mg injections plus MTX capsules (group 3) or golimumab 100 mg injections plus MTX capsules (group 4)2
This study assessed Changes in RAMRIS scores from baseline to weeks 12 and 24 in wrist plus MCP synovitis (p<0.001) and bone oedema (p=0.003).2


Simponi+MTX Reduce the signs and symptoms of RA Slow down the damage to bones and joints Improve physical function.4
MTX, Methotrexate. RA, Rheumatoid Arthritis. DAS, Disease Activity Score. MCP, metacarpophalangeal joint. TB, Tuberculosis.
TNFs, Tumor Necrosis Factors.
References:
1- Keystone EC, Genovese MC, Klareskog L, et al; GO-FORWARD Study. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GOFORWARD Study. Ann Rheum Dis. 2009 Jun; 68 (6) :789-96. 2- Conaghan PG, Emery P, Østergaard M, et al. Assessment by MRI of inflammation and damage in rheumatoid arthritis patients with methotrexate inadequate response receiving golimumab: results of the GO-FORWARD trial. Ann Rheum Dis. 2011 Nov; 70 (11) :1968-74. 3- Kay J, Fleischmann R, Keystone E, et al. Five-year Safety Data from 5 Clinical Trials of Subcutaneous Golimumab in Patients with Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis. J Rheumatol. 2016 Dec; 43 (12) :2120-2130. 4-Ministry of Health Simponi approved insert Leaflet 6-12-2020. 5- Keystone EC, Genovese MC, Hall S, et al. Safety and Efficacy of Subcutaneous Golimumab in Patients with Active Rheumatoid Arthritis despite Methotrexate Therapy: Final 5-year Results of the GO-FORWARD Trial. J Rheumatol. 2016 Feb; 43(2) :298-306.
CP-226097
