IMBRUVICA® IN chronic lymphocytic leukaemia (CLL): experience you can rely on1-5


Count on first-line efficacy
with 8 out of 10 patients alive and progression-free at 2 years1*
Data from 17.3 months' (11.9-25.6) median duration on ibrutinib (n=84) were presented at ASH 2020, reinforcing IMBRUVICA®'s use at first-line in routine practice in patients with high-risk CLL.1
84.5%
estimated Progression-Free Survival (PFS) at 24 months1
Low TREATMENT discontinuation RATE
and a well-established tolerability profile.1
4.76%
discontinued due to toxicity1*

Biggest IMBRUVICA® RWE study to date:
Longer times to next treatment
vs chemoimmunotherapy regardless of cytogenetic abnormalities2†
ASH 2020 also saw IMBRUVICA® continue to lead in generating real-world evidence (RWE) for targeted therapies. In the biggest RWE study to date (n=516) comparing outcomes in high-risk CLL patients on first-line IMBRUVICA® vs chemoimmunotherapy (CIT), patients were:2
54%
less likely to start a new treatment vs CIT (P<0.01)‡

Sustained efficacy in high-risk patients with TP53 aberrations3§
First-line chemoimmunotherapy is associated with a poor prognosis in patients with the del(17p) or TP53 mutation. In a four-year (48 months) follow up study (n=89), first-line IMBRUVICA® took strides to overcome the challenge facing this high-risk population.3§
Chemoimmunotherapy at three years in patients with del(17p) (n=51):6
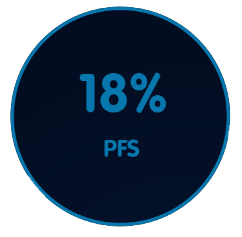
In a phase III study, at three years with chemoimmunotherapy (fludarabine, cyclophosphamide, and rituximab)6
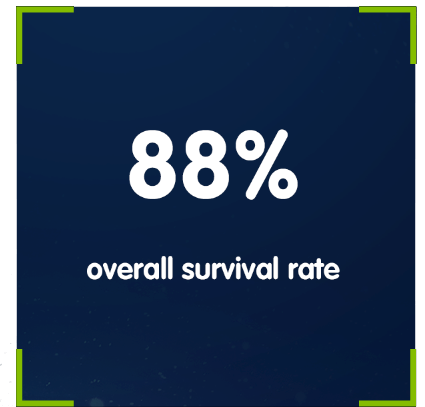
First-line IMBRUVICA® at four years in patients with del(17p) or the TP53 mutation (n=89):3




In a cross-trial comparison of a pooled analysis across 4 clinical trials, involving a mix of physically fit and unfit patients with a pooled median age of 65 years3

experience you can rely on:
Longest follow-up of any targeted agent in CLL with up to 79 months’ follow-up (n=498), confirming extended PFS with IMBRUVICA®-based treatment in patients with or without high-risk genomic features4II


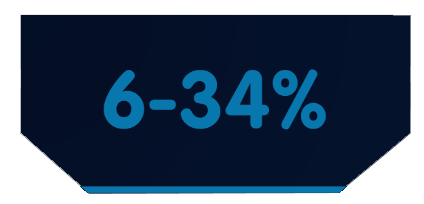
PFS rates were significantly higher with IMBRUVICA®-based treatment compared to chlorambucil-based treatment across high-risk genomic subgroups(95% CI: 0.14-0.25)4¶
PFS at 42 months with chlorambucil-based treatment
Take control with IMBRUVICA®
- Choose once-daily IMBRUVICA® and challenge the need for chemotherapy7-13
- Unsurpassed long-term survival for patients with B-cell malignancies14-19
- Patients can rely on the well-established long-term tolerability of IMBRUVICA®7,14-16,20,21
- Experience and trust for patients with B-cell malignancies7-9,17,20,22-29
Only IMBRUVICA® is backed by the experience and evidence that comes with treating over 200,000 patients in multiple indications (CLL, R/R MCL, WM) with up to 8 years of follow-up in clinical trials and in the real world4,19,30-32
* An observational, retrospective study describing the clinical outcomes of patients with treatment-naive CLL receiving IMBRUVICA® as first-line treatment (n=84) in routine clinical practice in Spain. 91.7% of patients had at least 1 high risk molecular cytogenetic factor (uIGHV, TP53 abnormalities, del11q or complex karyotype). The median duration of exposure to IMBRUVICA® was 17.3 (11.9-25.6) months. Dose reduction of IMBRUVICA® occurred in 17/84 (20.2%) patients, 8/84 (9.52%) due to toxicity (4 haematologic toxicity and 4 non-haematologic toxicity). 27/84 (32.1%) patients had temporary interruption of treatment. 49/84 (58.3%) patients developed at least one adverse event.1
† Study describing treatment patterns and time to next treatment in high risk and non-high risk patients with CLL receiving IMBRUVICA® or CIT as 1L therapy. Medical records were abstracted for adult high risk and non-high risk CLL patients from 40 US clinical practices (68% community; 32% academic), who initiated first-line IMBRUVICA® or CIT between 14th Febuary 2014 – 31st December 2016. Cytogenetic abnormalities (high risk) were defined as having one or more of the following prior to 1L initiation: del(17p), del(11q), TP53 mutation, unmutated IGHV, or complex karyotype. No significant differences were noted in time to next treatment between high risk and non-high risk IMBRUVICA® groups (p=0.06).2
‡ Median duration of first-line HR IMBRUVICA® was 28.6 (1-58.1) months vs 1L HR CI 5.5 (0.5-38.4).2
§ Data for first-line IMBRUVICA® treatment in patients with TP53 aberrations were pooled across 4 clinical trials in CLL/SLL: PCYC-1122e (n=34), PCYC-1130 (n=18), ECOG1912 (n=26), and RESONATE-2 (n=11). Long-term PFS (assessed by investigator), OS, and safety are reported.3
II This integrated analysis of patients undergoing first-line IMBRUVICA® treatment, with up to 79 months' follow-up. This analysis across two phase III studies demonstrated the efficacy of first-line IMBRUVICA® irrespective of cytogenetic and mutational risk features, including those with unmutated IGHV, NOTCH1 mutation, and those with the highest risk classification of del(17p)/TP53 mutation/BIRC3 mutation. In RESONATE-2, patients aged ≥65 years without del(17p) were randomised to IMBRUVICA® or chlorambucil. In iLLUMINATE, patients aged ≥65 years, or <65 years with coexisting conditions or del(17p)/TP53 mutation, were randomised to IMBRUVICA®-obinutuzumab or chlorambucil-obinutuzumab.4
IMBRUVICA® as a single agent or in combination with rituximab or obinutuzumab is indicated for the treatment of adult patients with previously untreated chronic lymphocytic leukaemia (CLL).5
¶ TPh3, SF3B1, IGHV, BIRC3, SF3B1, NOTCH1 and XPO1.
References
1. Costa PA et al. Blood 2020; 136 (Supplement 1): 32–33.
2. Huang Q et al. Clinical Outcomes Among RW patients with CLL Initiating 1L Ibrutinib or CIT Stratified By Risk Status: Results from a US Retrospective Chart Review Study. Oral and Poster Abstracts presented at 62nd ASH Annual Meeting & Exposition; 5-8 December 2020; all-virtual. #905.
3. Allan JN et al. Long-Term Efficacy of 1L Ibrutinib Treatment for CLL With 4 Years of Follow-Up in Patients With TP53 Aberrations (del(17p) or TP53 Mutation): A Pooled Analysis From 4 Clinical Trials. Oral and Poster Abstracts presented at 62nd ASH Annual Meeting & Exposition; 5-8 December 2020; all-virtual. #642.
4. Burger JA et al. Outcomes of 1L Ibrutinib in Patients with CLL/SLL and High-Risk Genomic Features with up to 6.5 Years Follow-up: Integrated Analysis of Two Phase 3 Studies (RESONATE-2 and iLLUMINATE). Oral and Poster Abstracts presented at 62nd ASH Annual Meeting & Exposition; 5-8 December 2020; all-virtual. #642.
5. IMBRUVICA® Summary of Product Characteristics. Janssen-Cilag International NV. 2020.
6. Hallek M et al. Lancet 2010; 376(9747): 1164-1174.
7. Burger JA et al. Leukemia 2020; 34(3): 787-798.
8. Moreno C et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab as first-line treatment in patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: up to 4 years of extended follow-up from the phase 3 iLLUMINATE study. Poster presented at the XVIII International Workshop on CLL (iwCLL) Meeting; 20-23 September 2019; Edinburgh, United Kingdom. #2069.
9. Woyach JA et al. N Engl J Med 2018; 379(26): 2517–2528.
10. Eichorst B et al. Ann Oncol 2015; 26(5): v78–v84.
11. Grupo Español de Leucemia Linfocítica Crónica (Spanish clinical guidelines for the management of CLL). 3rd edition. February 2019.
12. Wendtner C-M et al. Onkopedia leitlinien. Chronische Lymphatische Leukämie (CLL, Onkopedia guidelines, chronic lymphocytic leukaemia). April 2019.
13. NCCN clinical practice guidelines in oncology (NCCN guidelines®). Chronic lymphocytic leukemia/small lymphocytic lymphoma. Version 2.2021. December 2020.
14. Byrd JC et al. Up to 7 years of follow-up of single-agent ibrutinib in the phase 1b/2 PCYC-1102 trial of first line and relapsed/refractory patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Abstract presented at the 60th ASH Annual Meeting & Exposition; 1–4 December 2018; San Diego, CA, USA. #3133.
15. Byrd JC et al. Up to 7 years of follow-up of single-agent ibrutinib in the phase 1b/2 PCYC-1102 trial of first line and relapsed/refractory patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Poster presented at the 60th ASH Annual Meeting & Exposition; 1–4 December 2018; San Diego, CA, USA. #3133.
16. Treon SP et al. Ibrutinib monotherapy produces long-term disease control in previously treated Waldenstrom’s macroglobulinemia: final report of the pivotal trial. Oral presentation at the 15th International Conference on Malignant Lymphoma (ICML); 18–22 June 2019; Lugano Switzerland. #135.
17. Buske C et al. Ibrutinib treatment in Waldenström’s macroglobulinaemia : follow-up efficacy and safety from the iNNOVATE™ study. Oral presentation at the 60th ASH Annual Meeting and Exposition; 1–4 December 2018; San Diego, CA, USA.
18. Rule S et al. Long-term outcomes with ibrutinib versus the prior regimen: a pooled analysis in relapsed/refractory (R/R) mantle cell lymphoma (mcl) with up to 7.5 years of extended follow-up. Abstract presented at the 61st ASH Annual Meeting & Exposition; 7−10 December 2019; Orange County Convention Center (OCCC), Orlando, FL, USA. #1538.
19. Rule S et al. Long-term outcomes with ibrutinib versus the prior regimen: a pooled analysis in relapsed/refractory (R/R) mantle cell lymphoma (mcl) with up to 7.5 years of extended follow-up. Poster presented at the 61st ASH Annual Meeting & Exposition; 7−10 December 2019; Orange County Convention Center (OCCC), Orlando, FL, USA. #1538.
20. Munir T et al. Am J Hematol 2019; 94(12): 1353-1363.
21. O’Brien S et al. Clin Lymphoma Myeloma Leuk 2018; 18(10): 648-57.e15.
22. Rule S et al. Leukemia 2018; 32(8): 1799-1803.
23. Fraser G et al. Final 5-Year updated results from a Phase 3 Study (HELIOS) of ibrutinib plus bendamustine and rituximab (BR) in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Poster presented at the XVIII International Workshop on CLL (iwCLL) Meeting; 20-23 September 2019; Edinburgh, United Kingdom. #2021.
24. Slama B et al. French ibrutinib observational study (FIRE): real-world study of ibrutinib treatment for mantle cell lymphoma (MCL) in France. Abstract at the 24th Annual Congress of the European Hematology Association; 13–16 June 2019; Amsterdam, Netherlands. #PS1264.
25. Slama B et al. French ibrutinib observational study (FIRE): real-world study of ibrutinib treatment for mantle cell lymphoma (MCL) in France. Poster at the 24th Annual Congress of the European Hematology Association; 13–16 June 2019; Amsterdam, Netherlands. #PS1264.
26. Janssens A et al. Effectiveness and safety of ibrutinib for chronic lymphocytic leukemia (CLL) in routine clinical practice: interim analysis (IA) of the Belgian ibrutinib real-world data (BIRD) study. Abstract at the 24th Annual Congress of the European Hematology Association; 13–16 June 2019; Amsterdam, Netherlands. #PF384.
27. Janssens A et al. Effectiveness and safety of ibrutinib for chronic lymphocytic leukemia (CLL) in routine clinical practice: interim analysis (IA) of the Belgian ibrutinib real-world data (BIRD) study. Poster at the 24th Annual Congress of the European Hematology Association;
13–16 June 2019; Amsterdam, Netherlands. #PF384. 28. Aarup K et al. Real-world outcomes for 205 Danish patients with chronic lymphocytic leukemia treated with ibrutinib. Abstract presented at the 61st ASH Annual Meeting & Exposition; 7−10 December 2019; Orange County Convention Center (OCCC), Orlando, FL, USA. #1767.
29. Follows G et al. UK CLL forum 5 Year update on 315 relapsed refractory CLL patients treated with ibrutinib in 66 UK and Ireland centres. Abstract presented at the 61st ASH Annual Meeting & Exposition; 7−10 December 2019; Orange County Convention Center (OCCC), Orlando, FL, USA. #1767. 30. Byrd JC et al. Clin Cancer Res 2020; 26(15): 3918–3927.
31. Janssen Data on File. Ibrutinib – global number of cumulative patients treated with Ibrutinib since launch. EMEA-SR-1492. July 2020. 32. Treon SP et al. J Clin Oncol 2020; Epub ahead of print.
CP-20345