Withdrawal from TREVICTA® was associated with delayed time to relapse relative to that of INVEGA SUSTENNA® or oral paliperidone

that of INVEGA SUSTENNA® or ORAL paliperidone.5
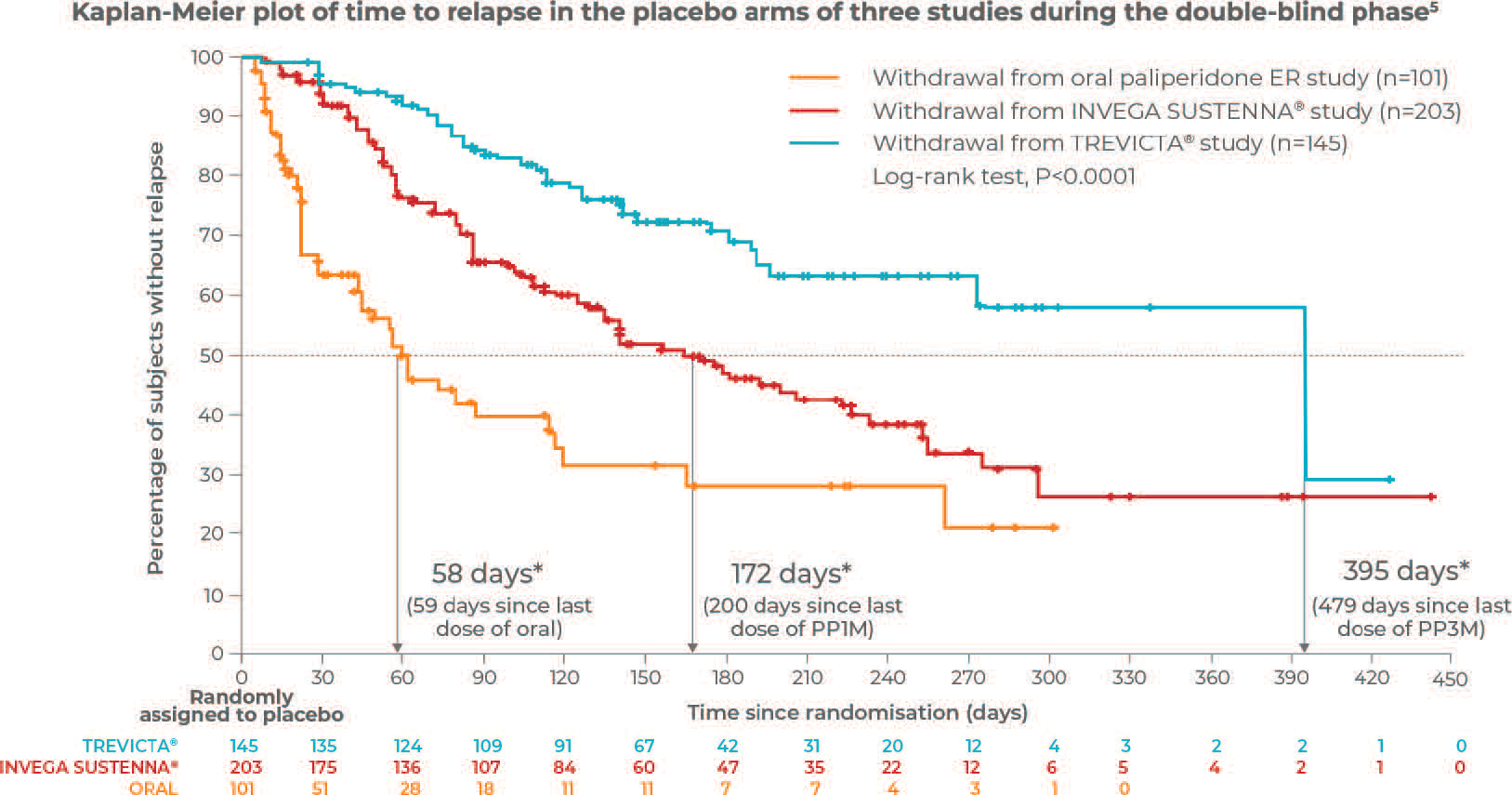
Adapted from Weiden P et al. 2017.
* Values shown for estimated median time to relapse correspond to the time since patients were randomised onto placebo following being symptomatically stable on either oral paliperidone ER, INVEGASUSTENNA® or TREVICTA® during the open-label phase.5
ER, extended release.
i. Patients who are adequately treated with 1-monthly paliperidone palmitate injectable (preferably for four months or more) and do not require dose adjustment may be switched to 3-monthly paliperidone palmitate
injection.2
This is a post hoc exploratory analysis to compare times to first relapse in adults with schizophrenia after double-blind discontinuation from ORAL paliperidone, once-monthly paliperidone palmitate (PP1M), or once-every-3-months paliperidone palmitate (PP3M).5
This analysis used data from similarly designed, randomized, double-blind, placebo-controlled, relapse prevention studies with ORAL paliperidone, PP1M, and PP3M.5
Briefly, men and women aged 18–65 years (≤ 70 years in the PP3M trial) were eligible if they had a schizophrenia diagnosis according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
(DSM-IV), criteria for ≥ 1 year before screening.5
References
1. INVEGA® Ministry of Health approved leaflet, 18/3/2019.
2. Risperdal® Ministry of Health approved leaflet, 16/12/2019.
3. INVEGA SUSTENNA® Ministry of Health approved leaflet, 18/3/2019.
4. TREVICTA® Ministry of Health approved leaflet.19/5/2019.
5. Weiden PJ, Kim E, Bermak J, Turkoz I, Gopal S, Berwaerts J. Does Half-Life Matter After Antipsychotic Discontinuation? A Relapse Comparison in Schizophrenia With 3 Different Formulations of Paliperidone. J Clin Psychiatry. 2017;78(7):e813-e820.
CP-253621