Easy Management of Skin Rash

We would like to share with you the below information regarding

Skin rash associated with ErleadaTM apalutamide was most commonly

The median days to onset of skin rash was

with a range of

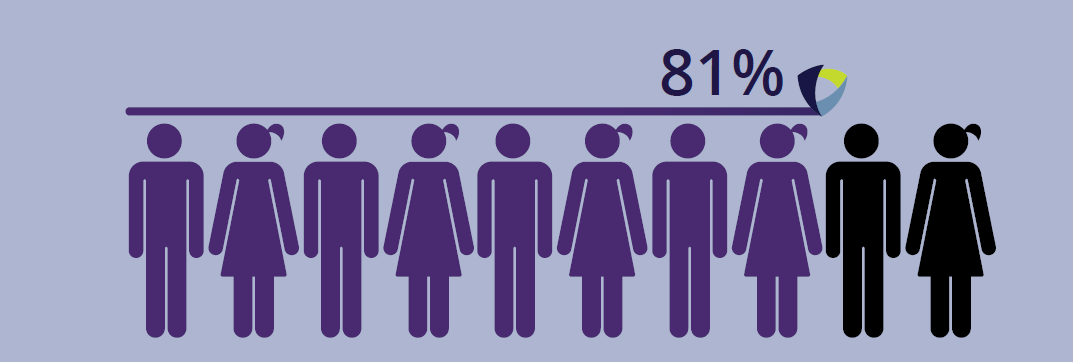
of patients had resolution of rash with a median of

to resolution.
skin rash (24% of any grade and 5% Grade 3).2
Grade 3 skin rashes (defined as covering >
30% BSA**) were reported with ErleadaTM
apalutamide treatment in 5.2% of patients.2
Medicinal products utilized included topical
corticosteroids, systemic corticosteroids and
oral anti-histamines. Among patients with skin
rash, dose interruption occurred in 28% and
dose reduction occurred in 12%.2
Skin rash recurred in approximately half
of patients who were re-challenged. Skin
rash led to ErleadaTM apalutamide treatment
discontinuation in 9% of patients who
experienced skin rash.2
References:
1-Ministry Of Health Erleada approved leaflet 3/2020
2- SUMMARY OF PRODUCT CHARACTERISTICS (SMPC), available at https://www.ema.europa.eu/en/documents/product-information/erleada-epar-product-information_en.pdf, last accessed at 9/11/2020
Abbreviations:
nmCRPC* non-metastatic castration-resistant prostate cancer.
BSA** Body Surface Area.
Abbreviated prescribing Information of Erleada
INDICATIONS:
Erleada is a cancer medicine that contains the active substance apalutamide. It is used to treat adult men with prostate cancer that:
- has metastasised to other parts of the body and still responds to medical or surgical treatments that lower testosterone (also called hormone-sensitive prostate cancer).
- has not metastasised to other parts of the body and no longer responds to medical or surgical treatment that lowers testosterone (also called castration-resistant prostate cancer).
DOSE:
How much to take
The recommended dose is 240 mg (four 60 mg tablets) once a day.
CONTRAINDICATIONS:
Do not take Erleada if
you are allergic to apalutamide or any of the other ingredients of this medicine.
you are a woman who is pregnant or may become pregnant.
Do not take this medicine if any of the above apply to you. If you are not sure, talk to your doctor or pharmacist before taking this medicine.
Other medicines and Erleada:
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This is because Erleada can affect the way some medicines work. Also,some other medicines can affect the way Erleada works.
Tell your doctor if you are taking medicines that:
• lower high fat levels in the blood (e.g. gemfibrozil).
• treat bacterial infections (e.g. moxifloxacin, clarithromycin).
• treat fungal infections (e.g. itraconazole, ketoconazole).
• treat HIV infection (e.g. ritonavir, efavirenz, darunavir).
• treat anxiety (e.g. midazolam, diazepam).
• treat epilepsy (e.g. phenytoin, valproic acid).
• treat gastroesophageal reflux disease (conditions where there is too much acid in the stomach) (e.g. omeprazole).
• prevent blood clots (e.g. warfarin, clopidogrel, dabigatran, etexilate).
• treat hay fever and allergies (e.g. fexofenadine).
• lower cholesterol levels (e.g. ‹statins› such as rosuvastatin, simvastatin).
• treat heart conditions or lower blood pressure (e.g. digoxin, felodipine).
• treat heart rhythm problems (e.g. quinidine, disopyramide, amiodarone, sotalol, dofetilide,ibutilide).
• treat thyroid conditions (e.g. levothyroxine).
• treat gout (e.g. colchicine).
• lower blood glucose (e.g. repaglinide).
• treat cancer (e.g. lapatinib, methotrexate).
• treat opioid addiction or pain (e.g. methadone).
• treat serious mental illnesses (e.g. haloperidol).
WARNING AND PRECAUTIONS:
Talk to your doctor or pharmacist before taking this medicine if:
• you have ever had fits or seizures
• you are taking any medicines to prevent blood clots (e.g. warfarin, acenocoumarol)
• you have any heart or blood vessel conditions, including heart rhythm problems (arrhythmia).
- Falls have been observed in patients taking Erleada. Take extra care to reduce your risk of a fall.
- Broken bones have been observed in patients taking Erleada.
- Blockage of the arteries in the heart that can lead to death has happened in some people during treatment with Erleada.
- Your healthcare provider will monitor you for signs and symptoms of heart problems during your treatment with Erleada.Call your healthcare provider or go to the nearest emergency room right away if you get chest pain or discomfort at rest or with activity or shortness of breath during your treatment with Erleada.
- If you are taking any medicines, talk to your doctor or pharmacist to see if they are associated with an increased risk of seizure, bleeding or heart condition.
- If any of the above apply to you talk to your doctor or pharmacist before taking Erleada.
SPECIAL POPULATION:
Children and adolescents
This medicine is not for use in children and adolescents under 18 years of age.
If a child or young person accidentally takes Erleada:
- go to the hospital straight away.
- take this package leaflet with you to show to the emergency doctor.
Pregnancy and contraception information for men and women
Information for women:
Erleada must not be taken by women who are pregnant, may become pregnant, or who are breastfeeding.
Erleada may harm your unborn baby.
Information for men -follow this advice during treatment and for 3 months after stopping:
• If you are having sex with a pregnant woman -use a condom to protect the unborn baby.
• If you are having sex with a woman who can become pregnant –use condom and another highly effective method of contraception.
- Use contraception during treatment and for 3 months after stopping. Talk to your doctor if you have any questions about contraception.
- Erleada may reduce male fertility.
Driving and using machines:
This medicine is not likely to affect you being able to drive and use any tool or machine . The side effects for Erleada include seizures. If you are higher risk of seizures ( see Section 3 Warning and precautions), talk to your doctor.
Possible side effects:
Like all medicines, this medicine can cause side effect , although not everybody get them.
Stop using Erleada and seek medical attention immediately if you notice any of the following symptoms:
- Reddish non-elevated, target-like or circular patches on the trunk, often with central blisters, skin peeling, ulcers of mouth, throat, nose, genitals and eyes. These serious skin rashes can be preceded by fever and flu-like symptoms (toxic epidermal necrolysis).
Serious side effects:
Tell your doctor straight away if you notice any of the following serious side effect -your doctor may stop treatment:
- fit or seizure -this is uncommon(may affect up to 1 in 100 people) Your healthcare provider will stop Erleada if you have a seizure during treatment.
- falls or fractures (broken bones) these are very common (may affect more than 1 in 10 people) Your healthcare provider may monitor you more closely if you are at risk for fractures.
- heart disease -this is common (may affect up to 1 in 10 people) your healthcare provider will monitor you for signs and Symptoms of heart problems during your treatment. Call your healthcare provider or go to the nearest emergency room right away if you get chest pain or discomfort at restor with activity or shortness of breathing during your treatment with Erleada.
Other side effects include
Very common (may affect more than I in 10 people).
• feeling very tired
• joint pain
• skin rash
• Decreased Appetite
• high blood pressure
• hot flush
• diarrhea
• broken bones
• falls
• weight loss
Common (may affect up to l in 10 people):
• muscle spasms
• itching
• change in sense of taste
• blood test showing high level of cholesterol in the blood
• blood test showing high level of a type of fat called “triglycerides” in the blood
• under-active thyroid which can make you feel more tired and have difficulty getting started in the morning, and blood tests may also show an under-active thyroid.
Not known (frequency cannot be estimated from the available data):
abnormal heart tracing on an ECG (electrocardiogram).)
life-threatening rash with blisters and peeling over much of the body (toxic epidermal necrolysis).
- Ministry Of Health Erleada approved leaflet 11/2020.
- Always read the full prescribing information.
- Healthcare professionals are asked to report any suspected adverse reactions pharmacovigilance center (EPV).
.png?width=300&height=217&format=png&quality=50)

CP-224880